Homocysteine
 What is Homocysteine?
What is Homocysteine?
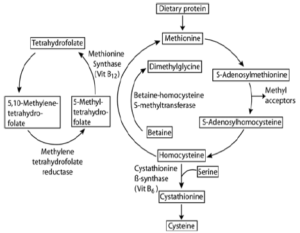
Homocysteine (Hcy) is a sulfhydryl-containing amino acid produced by intracellular demethylation of the essential amino acid methionine (Met). It is not used to make proteins but as an intermediate in methionine metabolism.
What does homocysteine do?
Serum Homocysteine isoforms are reduced to free Homocysteine, and then exogenous S-adenosylmethionine (SAM) methylates Homocysteine to form SAH and Met, a reaction catalyzed by Homocysteine methyltransferase. SAH is then hydrolyzed to Homocysteine and adenosine (ADO) by SAH hydrolase. ADO is immediately hydrolyzed into inosine and ammonia, which reacts with glutamate dehydrogenase with concomitant conversions of NADH to NAD+ measured at ΔA340nm.
Homocysteine continuously cycles through this reaction to significantly amplify the detection signal. B vitamins are important cofactors in this metabolic pathway. When methionine levels are low, methyltetrahydrofolate (from folic acid) donates methyl groups to generate methionine from Homocysteine, and vitamin B12 is also necessary for the methionine synthase-catalyzed remethylation of homocysteine into methionine. When there is excess methionine, the biologically active form of vitamin B6 catalyzes homocysteine transulfuration to cystathionine and ultimately to cysteine (excess of which is eliminated by the body).
Homocysteine and Cardiovascular Disease
Homocysteine mediates adverse effects on the blood vessels through diverse pathways:
- homocysteine isoforms directly damage the blood vessel walls11
- interactions between homocysteine and low-density lipoprotein (LDL) cholesterol contribute to subendothelial plaque genesis3
- post-translational modifications of LDL particles, promoting the formation of foam cells and plaque progression12
- increased peroxide production and reduced glutathione peroxidase activity increase endothelial cell damage13
- reduced nitric oxide availability results in vasoconstriction11
- activation of monocytes leads to cytokine secretion and inflammation3,14
- platelet activation and aggregation, reduced thrombomodulin expression, depressed protein-C activity, and tissue plasminogen activator cause dysregulation of the coagulation system.3,15,16
Hyperhomocysteinemia is also associated with impaired hepatic capacity to synthesize apoA-I, thereby lowering HDL particle concentrations.17
Although the association between Hcy and vascular disease is widely recognized, elevated Hcy has also been associated with the following disorders and diseases:
- Deficiency of folic acid or vitamins B6/B12: Elevated homocysteine levels are most commonly caused by low levels of one or all of these B vitamins. A normal homocysteine excludes significant B vitamin deficiency.15,18
- Liver disease: Elevated Hcy and low levels of SAM are associated with liver toxicity and cirrhosis.19,20 Homocysteine may cause liver damage, leading to the formation of fibrin, clots, and vascular complications.21
- Renal disease: homocysteine levels rise during renal insufficiency due to inadequate homocysteine filtration.22,23
- Thyroid conditions: Elevated homocysteine levels may contribute to accelerated heart disease in patients with concomitant hypothyroidism.24
- Alzheimer’s disease and dementia: Elevated homocysteine levels have been associated with Alzheimer’s disease incidence, indicating impaired methylation in brain tissue.25,26,27
- Depression: Low serum folic acid levels have been associated with depression in women.28,29 Of interest, low levels of serum folate reduce the efficacy of fluoxetine (Prozac®, an antidepressant),28 while Vitamin B6 may alleviate depression.30
- Erectile dysfunction: A case study described a patient with erectile dysfunction who had a genetic defect causing elevated serum homocysteine levels. Although the patient did not initially respond to treatment with sildenafil (Viagra®), subsequent supplementation with 5 mg of folic acid and 1 mg of vitamin B12 resulted in successful treatment of his erectile dysfunction with sildenafil.31 Homocysteine has been shown to reduce production of nitric oxide, a vasodilator that increases blood flow to organs such as the penis.32
- Ocular disease: homocysteine’s ability to damage blood vessels extends to the ocular microvasculature. Elevated homocysteine levels are associated with ocular diseases such as glaucoma and macular degeneration. Mean homocysteine levels in patients with central retinal vein occlusion were 11.6 μmol/L compared with 9.5 μmol/L in control subjects.33
Additional risk factors for elevated homocysteine levels include advanced age (often due to vitamin B12 deficiency owing to increased malabsorption with age), male sex, genetic defects of homocysteine metabolizing enzymes, diabetes mellitus, smoking, coffee intake, hyperproliferative conditions (e.g. psoriasis, rheumatoid arthritis), malignancies and drugs that affect homocysteine metabolism (e.g., some lipid-modifying drugs, anticonvulsants, folate and B12 antagonists, metformin).6,18,34,35
Should we lower Homocysteine?
The many links between elevated plasma homocysteine and diseases that afflict the elderly point to the existence of a common denominator that may be responsible for these diseases. Whether this denominator is homocysteine itself or whether homocysteine is merely a biomarker remains to be determined.
Since multiple clinical trials have demonstrated no cardiovascular event reductions despite lowering homocysteine using folic acid and B vitamin supplementation, the benefit of homocysteine-lowering strategies has been debated.4,6,15,34,36,37
However, the development of an atherosclerotic plaque takes a long time, typically 30-40 years from initial development to a clinical event. If homocysteine were a true causal contributor to this process, the time required to show a beneficial intervention effect of lowering homocysteine could take much longer than the < 5 years of follow-up characterizing the B-vitamin intervention trials.15
Thus it is possible that the homocysteine-lowering trials, although studying large numbers of patients, have been underpowered simply because the duration of follow-up has been too short in relation to the development time-frame for atherosclerotic disease.15,36 In support of this notion, a meta-analysis of the effect of B vitamins on stroke showed a full absence of any effect in short term studies (< 36 months) but a statistically significant 29% reduction in the studies with at least 36 months of follow-up.38
A large meta-analysis of more than 90 genetic and prospective studies calculated that lowering homocysteine levels by 3 µmol/L (by increased folic acid intake) would reduce the risk of ischemic heart disease by 16%, DVT by 25% and stroke by 25%.8 Lowering homocysteine levels may also increase nitrous oxide availability and reduce endothelial dysfunction.15,37 But the debate over whether plasma homocysteine can be considered a modifiable risk factor for atherosclerosis still rages.15,36
In particular, it is still unclear whether endogenous variations of plasma homocysteine within normal range are accompanied by clinically significant variations in endothelial dysfunction, oxidative stress, or the overall thrombotic state.15
Beneficial Dietary Modifications
Ingestion of Met may increase homocysteine levels since homocysteine is a Met metabolite. Indeed, homocysteine levels have been increased in experimental protocols through Met supplement ingestion.39 Foods rich in Met such as meat and eggs have been associated with increased risk of heart disease, although causality between Met content and CVD has not been established.
Diets high in fruits and vegetables that contain folic acid, beta-carotene, and vitamin C lowered homocysteine levels: in one study, healthy people consumed diets containing either one pound of fruits and vegetables or 3½ ounces of fruits and vegetables daily; homocysteine levels were 11% lower in the former group as compared with the latter after four weeks.40
Also of interest, replacing a breakfast of refined rice with whole-grain and legume powder significantly lowered homocysteine levels in men with cardiovascular disease (CVD).41 People with elevated homocysteine are typically advised to eat less processed foods, meat, and saturated fat to lower the risk of heart disease.
Beneficial Lifestyle Modifications
Cigarette smoking and coffee consumption are associated with increased homocysteine levels,42 an observation consistent with the association between smoking and caffeine consumption and increased risk of both CVD and osteoporosis. The correlation between coffee intake and increased homocysteine has been confirmed in some,43 but not all, studies.44 T
o exemplify the effects of lifestyle modification on homocysteine levels, a diverse group of people participated in a weeklong program that included a strict vegan diet, stress management, spirituality enhancement sessions, group support, and exclusion of tobacco, alcohol, and caffeine.45 B vitamin supplements known to reduce plasma homocysteine levels were not provided. Mean homocysteine levels declined by 13% after one week of lifestyle modification.
Thus a whole diet approach high in fruit and vegetables with high natural folate content is recommended to decrease plasma homocysteine levels, with the added benefit of increasing the intake of other important nutrients as well.
Beneficial Supplementations
Vitamins B6, B12, and folic acid are essential cofactors in homocysteine metabolism to Met and cysteine, and therefore supplementation has consistently lowered plasma homocysteine.3,6,15,18,37 Oral administration of folic acid (0.5-5 mg/day) reduces fasting homocysteine levels by 25-30%, while supplementation with vitamin B12 (0.02-1 mg/day) yields an additional 7% reduction in homocysteine levels.46
Folic acid supplementation lowers homocysteine levels most significantly in healthy individuals.47,48 However, a decrease of serum homocysteine of 3 µmol/L is achievable by daily intake of ~0.8 mg folic acid and should reduce risk of ischemic heart disease by 16%, DVT by 25% and stroke by 24%.8
In 1996, the FDA required that all enriched flour, rice, pasta, cornmeal, and other grain products contain 140 µg of folic acid per 3.5 ounces.49 This level of fortification has led to a measurable decrease in serum homocysteine levels.50
However, higher levels of folic acid fortification result in further homocysteine reductions, suggesting that the FDA-mandated supplementation is inadequate to optimally protect people against hyperhomocysteinemia. Therefore, if one wishes to optimally reduce homocysteine levels, despite the current lack of level I evidence supporting CV risk reduction,36,37 folic acid supplements should complement the FDA-mandated fortification program.
Vitamin B2 (riboflavin) supplementation (1.6 mg per day) lowered homocysteine levels by 22-40% in individuals with a genetic variant of an enzyme involved in folic acid metabolism (the 677C>T polymorphism of the methylenetetrahydrofolate reductase (MTHFR) gene.51 Approximately 10% of the population are homozygous for this genetic variant and could benefit from riboflavin supplementation.52,53
An alternative therapy is supplementation with methylated folic acid, the active form of folate. Specific methyl donors such as TMG/ betaine (1.5-6 g per day; 1.5 g usual standard diets) and choline (2 g per day or 2.6 g daily provided as 34 g of soy lecithin) may also lower homocysteine supplements, are other potential aids to the conversion of homocysteine to methionine (simultaneously lowering serum homocysteine). Doctors have traditionally considered supplementation with these nutrients only when vitamin B6, vitamin B12, and folate supplementation fail to reduce homocysteine to optimal levels, but they can be key methyl donors in the presence of MTHFR mutations which impair methylation ability.52
Despite the beneficial health effects of supplementation with B vitamins, it is important to note that there are possible dual effects on atherosclerosis, with the intrinsic beneficial effects of homocysteine-lowering being offset by detrimental effects of unmetabolized synthetic folic acid and/or stimulation of inflammation (and possibly proliferation) in existing atherosclerotic lesions by B vitamins.15,36 These factors may partly explain why clinical trials on homocysteine lowering failed to report improvement in CV risk and clinical outcome, and are currently under intense investigation. Following current recommendations, the target plasma homocysteine level is ≤ 10 µmol/L.15,18,34,36,57
In patients with CVD or high-risk for CVD events, a high homocysteine level may be used as a prognostic factor for CVD events and mortality:
- Mild to moderate hyperhomocysteinemia indicates high risk for CVD and usually stems from poor diet, mild folate/B12/B6 deficiency, hypothyroidism, impaired renal function, or certain drugs.15,18,34,57 When the cause of moderate hyperhomocysteinemia is established, then the best treatment is reversal of this cause.
- Increased tHcy combined with low B vitamin levels indicates a potential vitamin deficiency: supplement with a multivitamin containing moderate amounts of folic acid (0.2-0.8 mg), B6 (2-25 mg) and B12 (3-100 µg/day).18
- Switch to high-dose B vitamins only if homocysteine clearly remains elevated and there is no MTHFR mutation (in which case riboflavin or supplementary methyl donors such as 5-MTHF or betaine may lower tHcy).
- Intermediate hyperhomocysteinemia (fasting plasma tHcy 30-100 µmol/L) is usually the result of moderate/severe vitamin B12 or folate deficiency, homozygous enzyme defects in homocysteine metabolism, or renal failure.34,57 Again, diagnosis and reversal of the respective cause should be the main priority, while most patients respond well to folate alone or in combination with B12, B6 and possibly betaine. Use of all 4 supplements reduces homocysteine in people with renal failure. Notably, high doses of vitamin B12 are required for normalization of homocysteine levels after renal failure.23
- Severe vitamin B12 deficiency and homocystinuria are the main causes of severe hyperhomocysteinemia (fasting plasma tHcy >100 µmol/L) and should be treated accordingly (0.02-1 mg/day of B12, 5-25 mg/day of B6 and 1-5 mg/day of folic acid),18 since it is associated with an increased pro-thrombotic state.
Long-term treatment with high doses of folic acid may mask pernicious anemia caused by vitamin B12 deficiency. The high folate may correct the anemia but allow the neuropathy to progress undiagnosed, leading eventually to irreversible degeneration of the spinal cord. It is therefore important to measure B12 levels before the start of folate treatment and/or treat with folate and B12 together.
References
- Malinow MR, Nieto FJ, Szklo M, et al. Carotid artery intimal-medial wall thickening and plasma homocyst(e)ine in asymptomatic adults. The Atherosclerosis Risk in Communities Study. Circulation 1993; 87(4):1107-13.
- McCully KS. Homocysteine, folate, vitamin B6, and cardiovascular disease. JAMA 1998; 279(5):392-3.
- Manolescu BN, Oprea E, Farcasanu IC, et al. Homocysteine and vitamin therapy in stroke prevention and treatment: a review. Acta Biochimica Polonica 2010;57(4):467-477.
- Selhub J. The many facets of hyperhomocysteinemia: studies from the Framingham cohorts. J Nutr 2006;136:1726S-1730S.
- Christen WG, Ajani UA, Glynn RJ, Hennekens CH. Blood levels of homocysteine and increased risks of cardiovascular disease: causal or casual? Arch Intern Med 2000;160:422-434.
- Wierzbucki, AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res 2007;4:143-50.Garlick PJ. Toxicity of methionine in humans. J Nutr 2006;136:1722S-1725S
- Broekmans WM, Klopping-Ketelaars IA, Schuurman CR, et al. Fruits and vegetables increase plasma carotenoids and vitamins and decrease homocysteine in humans. J Nutr 2000;130:1578–83.
- Jang Y, Lee JH, Kim OY, et al. Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: randomized controlled clinical trial. Arterioscler Thromb Vasc Biol 2001;21:2065–71.
- Nygård O, Refsum H, Ueland PM, Vollset SE. Major lifestyle determinants of plasma total homocysteine distribution: the Hordaland Homocysteine Study. Am J Clin Nutr 1998;67:263–70.
- Stolzenberg-Solomon RZ, Miller ER 3rd, Maguire MG, et al. Association of dietary protein intake and coffee consumption with serum homocysteine concentrations in an older population. Am J Clin Nutr 1999;69:467–75.
- Stanger O, Weger M. Interactions of homocysteine, nitric oxide, folate and radicals in the progressively damaged endothelium. Clin Chem Lab Med 2003;41(11):1444-1454.
- Thomson MJ, Puntmann V, Kaski JC. Atherosclerosis and oxidant stress: the end of the road for antioxidant vitamin treatment? Cardiovasc Drugs Ther 2007;21:195-210.
- Weiss N, Heydrick SJ, Postea O, et al. Influence of hyperhomocysteinemia on the cellular redox state – impact of homocysteine-induced endothelial dysfunction. Clin Chem Lab Med 2003;41(11):1455-1461.
- El Oudi M, Aouni Z, Mazigh C, et al. Homocysteine and markers of inflammation in acute coronary syndrome. Exp Clin Cardiol 2010;15:e25-e28.
- Antoniades C, Antonopoulos AS, Tousoulis D, et al. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. European Heart J 2009;30:6-15.
- Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost 2005;3:1646-54.
- Liao, D, Tan, H, Hui, R, et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipopotein A-1 protein synthesis and enhancing HDL cholesterol clearance. Circ Res 2006; 99:598-606.
- Stanger O, Herrmann W, Pietrzik K, et al. Clinical use and rational management of homocysteine, folic acid, and B vitamins in cardiovascular and thrombotic diseases. Z Kardiol 2004;93:439-453
- Martínez-Chantar ML, García-Trevijano ER, Latasa MU, et al. Importance of a deficiency in S-adenosyl-L-methionine synthesis in the pathogenesis of liver injury. Am J Clin Nutr 2002;76(5):1177S-82S.
- Ventura P, Rosa MC, Abbati G, et al. Hyperhomocysteinaemia in chronic liver diseases: role of disease stage, vitamin status and methylenetetrahydrofolate reductase genetics. Liver Int 2005;25(1):49-56.
- de la Vega MJ, Santolaria F, González-Reimers E, et al. High prevalence of hyperhomocysteinemia in chronic alcoholism: the importance of the thermolabile form of the enzyme methylenetetrahydrofolate reductase (MTHFR). Alcohol 2001;25(2): 59-67.
- Friedman AN, Bostom AG, Selhub J, et al. The kidney and homocysteine metabolism. J Am Soc Nephrol 2001;12(10):2181-9.
- Righetti M, Tommasi A, Lagona C, et al. Effective homocysteine-lowering vitamin B treatment in peritoneal dialysis patients. Perit Dial Int 2004;24(4):373-7.
- Morris MS, Bostom AG, Jacques PF, et al. Hyperhomocysteinemia and hypercholesterolemia associated with hypothyroidism in the third US National Health and Nutrition Examination Survey. Atherosclerosis 2001;155(1):195-200.
- McCaddon A, Davies G, Hudson P, et al. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry 1998;13(4):235-9.
- Werder SF. Cobalamin deficiency, hyperhomocysteinemia, and dementia. Neuropsychiatr Dis Treat 2010;6:159-95.
- Hermann W, Obeid R. Homocysteine: a biomarker in neurodegenerative diseases. Clin Chem Lab Med 2011;49(3):435-441.
- Fava M, Borus JS, Alpert JE, et al. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry 1997;154(3):426-8.
- Beydoun MA, Shroff MR, Beydoun HA, Zonderman AB. Serum folate, vitamin B-12, and homocysteine and their association with depressive symptoms among U.S. adults. Psychosom Med 2010;72(9):862-73.
- Hvas AM, Juul S, Bech P, et al. Vitamin B6 level is associated with symptoms of depression. Psychother Psychosom 2004;73(6):340-3.
- Lombardo F, Sgrò P, Gandini L, Dondero F, Jannini EA, Lenzi A. Might erectile dysfunction be due to the thermolabile variant of methylenetetrahydrofolate reductase? J Endocrinol Invest 2004;27(9):883-5.
- Demir T, Comlekçi A, Demir O, et al. Hyperhomocysteinemia: a novel risk factor for erectile dysfunction. Metabolism 2006;55(12):1564-8
- Vine AK. Hyperhomocysteinemia: a new risk factor for central retinal vein occlusion. Trans Am Ophthalmol Soc 2000;98:493-503.
- Refsum H, Smith AD, Ueland PM, et al. Facts and recommendations about total homocysteine determinations: an expert opinión. Clin Chem 2004;50:3-32.
- Yaman H, Akgul EO, Kurt YG, et al. Plasma total homocysteine concentrations in a Turkish population sample. Acta Cardiol 2009;64(2):247-51.
- Smulders YM, Blom HG. The homocysteine controversy. J Inherit Metab Dis 2011;34:93-99.
- Clarke R, Halsey J, Lewington S, et al. B-Vitamin Treatment Trialists’ Collaboration. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med 2010;170(18):1622-31.
- Wang X, Qin X, Demirtas H, Li J, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 2007;369:1876-1882.
- Garlick PJ. Toxicity of methionine in humans. J Nutr 2006;136:1722S-1725S
- Broekmans WM, Klopping-Ketelaars IA, Schuurman CR, et al. Fruits and vegetables increase plasma carotenoids and vitamins and decrease homocysteine in humans. J Nutr 2000;130:1578–83.
- Jang Y, Lee JH, Kim OY, et al. Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: randomized controlled clinical trial. Arterioscler Thromb Vasc Biol 2001;21:2065–71.
- Nygård O, Refsum H, Ueland PM, Vollset SE. Major lifestyle determinants of plasma total homocysteine distribution: the Hordaland Homocysteine Study. Am J Clin Nutr 1998;67:263–70.
- Stolzenberg-Solomon RZ, Miller ER 3rd, Maguire MG, et al. Association of dietary protein intake and coffee consumption with serum homocysteine concentrations in an older population. Am J Clin Nutr 1999;69:467–75.
- Nieto FJ, Comstock GW, Chambless LE, Malinow RM. Coffee consumption and plasma homocyst(e)ine: results from the Atherosclerosis Risk in Communities Study. Am J Clin Nutr 1997;66:1475–85 [letter].
- DeRose DJ, Charles-Marcel ZL, Jamison JM, et al. Vegan diet-based lifestyle program rapidly lowers homocysteine levels. Prev Med 2000;30:225–33.
- Moens AL, Vrints Cj, Claeys MJ et al. Mechanisms and potential therapeutic targets for folic acid in cardiovascular disease. Am J Physiol Heart Circ Physiol 2008;294:H1971-H1977.
- Dierkes J, Kroesen M, Pietrzik K. Folic acid and vitamin B6 supplementation and plasma homocysteine concentrations in healthy young women. Int J Vitam Nutr Res 1998;68:98–103.
- Stein JH, McBride PE. Hyperhomocysteinemia and atherosclerotic vascular disease: pathophysiology, screening, and treatment. Arch Intern Med 1998;158:1301–6.
- Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Fed Regist 1996;61:8781–97.
- Jacques PF, Selhub J, Bostom AG, et al. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med 1999;340:1449–54.
- McNulty H, Dowey LR, Strain JJ, et al. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C>T polymorphism. Circulation 2006;113:74–80.
- Ueland PM, Hustad S, Schneede J, et al. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci 2001;22(4):195-201.
- dbSNP – Database for short genetic variations. http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=1801133. Accessed May 2011.
- Wilcken DEL, Wilcken B, Dudman NP, Tyrrell PA. Homocystinuria—the effects of betaine in the treatment of patients not responsive to pyridoxine. N Engl J Med 1983;309:448–53.
- Olthof MR, Brink EJ, Katan MB, Verhoef P. Choline supplemented as phosphatidylcholine decreases fasting and postmethionine-loading plasma homocysteine concentrations in healthy men. Am J Clin Nutr 2005;82:111–7.
- Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr 2003;133:4135–8.
- Ogbonna G, Gottermeier G, Fox LS. Non-fasting plasma total homocysteine reference interval using the Vitros homocysteine assay standardized against NIST SRM 1955.Clin Chim Acta 2008;388(1-2):225-7.