Support of Hormone Metabolism Healthy metabolism of exogenous and endogenous estrogens can be pivotal for hormonal balance. DIM promotes metabolism of estrogen into the favorable and protective 2-hydroxyestrone (2-OHE) metabolite versus production of 4-hydroxyestrone (4-OHE) and 16-alpha-hydroxyestrone (16-alpha- OHE) metabolites. DIM’s influence on 2-OHE production creates a more desirable ratio of 2-OHE to 16-alpha-OHE. Assessment of 2:16-alpha-OHE ratio appears to be useful in evaluating breast health. DIM has been studied for its role in supporting prostate health as well, by reducing dihydrotestosterone binding to androgen receptors.*
Support of Cell Function and Metabolism Orchestration of metabolism by the thyroid gland is dependent on hormone balance. DIM was found to target proteolytic enzymes (MMP-2 and MMP-9), thus supporting the normal function and activity of thyroid cells in vitro. Ongoing research reveals DIM’s positive role in regulation of gene expression, protein production, and cell function. Downregulation of certain proteins (survivin, Bcl-2, and cdc25A) and upregulation of protective proteins (NRF2 and cyclin-dependent kinase inhibitor p21waf1) promoted healthy cell growth.*
Antioxidant and Detoxification Support DIMension 3 provides support for both antioxidant and detoxification systems which, in turn, support cellular function and integrity. Antioxidant activity is crucial to counteracting oxidative molecules normally produced during phase I detoxification. Research on DIM suggests that it plays an important role in activating detoxification enzymes in human hepatocytes, further supporting biotransformation at a primary site in the body.*
Curcumin As the major curcuminoid found in turmeric, curcumin is valued for its promotion of antioxidant activity, support of metabolic detoxification, and modulation of cytokine production. Research studying genotoxic estrogen metabolites suggests that curcumin’s inhibitory effect on anchorage-independent growth and on CYP enzymes following dioxin exposure helps support healthy cell-life regulation in human embryonic kidney cells and normal prostate cells.*
BioPerine is a patented form of piperine, the main alkaloid from black and long pepper plants that has been found to effectively support the absorption of nutrients. After a dose of 2 g of curcumin, human serum levels of curcumin were either undetectable or very low. When the same dose was given along with 20 mg of piperine (4:1 ratio), there was a 2000% increase in the bioavailability of the curcumin without adverse effects.*
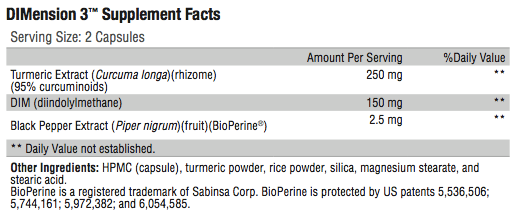
Directions:
Take two capsules daily, or as directed by your healthcare practitioner.
References:
- Bradlow HL. Review. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo. 2008 Jul-Aug;22(4):441-5. [PMID: 18712169]
- Herrmann S, Seidelin M, Bisgaard HC, et al. Indolo[3,2-b]carbazole inhibits gap junctional intercellular communication in rat primary hepatocytes and acts as a potential tumor promoter. Carcinogenesis. 2002 Nov;23(11):1861-8. [PMID: 12419834]
- Riby JE, Xue L, Chatterji U, et al. Activation and potentiation of interferon- gamma signaling by 3,3′-diindolylmethane in MCF-7 breast cancer cells. Mol Pharmacol. 2006 Feb;69(2):430-9. [PMID: 16267208]
- Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002 Apr;7(2):112-29. [PMID: 11991791]
- Cavalieri E, Frenkel K, Liehr JG, et al. Estrogens as endogenous genotoxic agents–DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;(27):75- 93. [PMID: 10963621]
- Im A, Vogel VG, Ahrendt G, et al. Urinary estrogen metabolites in women at high risk for breast cancer. Carcinogenesis. 2009 Sep;30(9):1532-5. [PMID: 19502596]
- Fares F, Azzam N, Appel B, et al. The potential efficacy of 3,3′-diindolylmethane in prevention of prostate cancer development. Eur J Cancer Prev. 2010 May;19(3):199-203. [PMID: 20010430]
- Le HT, Schaldach CM, Firestone GL, et al. Plant-derived 3,3’-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003 Jun 6;278 (23): 21136-45. [PMID: 12665522]
- Rajoria S, Suriano R, George A, et al. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in thyroid cancer. PLoS One. 2011 Jan 18;6(1):e15879. [PMID: 21267453]
- Ahmad A, Sakr WA, Rahman KM. Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr Drug Targets. 2010 Jun;11(6):652-66. [PMID: 20298156]
- Rahman KW, Li Y, Wang Z, et al. Gene expression profiling revealed survivin as a target of 3,3′-diindolylmethane-induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res. 2006 May 1;66(9):4952-60. [PMID: 16651453]
- Gross-Steinmeyer K, Stapleton PL, Liu F, et al. Phytochemical-induced changes in gene expression of carcinogen-metabolizing enzymes in cultured human primary hepatocytes. Xenobiotica. 2004 Jul;34(7):619-32. [PMID: 15672752]
- Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009 Jun;14(2):141-53. [PMID: 19594223]
- Choi H, Chun YS, Shin YJ, et al. Curcumin attenuates cytochrome P450 induction in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by ROS- dependently degrading AhR and ARNT. Cancer Sci. 2008 Dec;99(12):2518-24. [PMID: 19018768]
- Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998 May; 64 (4):353-6. [PMID: 9619120]